
Journal of Medicinal Chemistry: Targeting the Allosteric Pathway That Interconnects the Core-Functional Scaffold and the Distal Phosphorylation Sites for Specific Dephosphorylation of Bcl-2
Authors: Ziqian Wang, Ting Song*, Zongwei Guo, Keke Cao, Chao Chen, Yingang Feng, Hang Wang, Fangkui Yin, Sheng Zhou, Jian Dai, and Zhichao Zhang*
Abstract
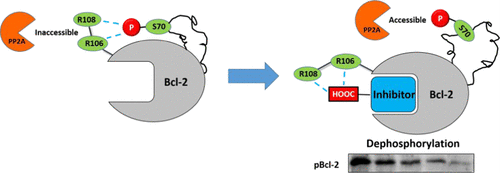
Protein phosphorylation is the most significant post-translational modification for regulating cellular activities, but site-specific modulation of phosphorylation is still challenging. Using three-dimensional NMR spectra, molecular dynamics simulations, and alanine mutations, we identified that the interaction network between pT69/pS70 and R106/R109 residues prevents the phosphorylation sites from exposure to phosphatase and subsequent dephosphorylation. A Bcl-2-dephosphorylation probe, S1-6e, was designed by installing a carboxylic acid group to a Bcl-2 inhibitor. The carboxyl group competitively disrupts the interaction network between R106/R109 and pT69/pS70 and subsequently facilitates Bcl-2 dephosphorylation in living cells. As a result, S1-6e manifests a more effective apoptosis induction in pBcl-2-dependent cancer cells than other inhibitors exhibiting a similar binding affinity for Bcl-2. We believe that targeting the allosteric pathways interconnecting the core-functional domain and the phosphorylation site can be a general strategy for a rational design of site-specific dephosphorylating probes, since the allosteric pathway has been discovered in a variety of proteins.
https://pubs.acs.org/doi/10.1021/acs.jmedchem.0c01290