
Biochemical Pharmacology: Bim transfer between Bcl-2-like protein and Hsp70 underlines Bcl-2/Hsp70 crosstalk to regulate apoptosis
Authors: Hong Zhang, Zongwei Guo , Yafei Guo , Ziqian Wang , Yao Tang , Ting Song , Zhichao Zhang
Abstract
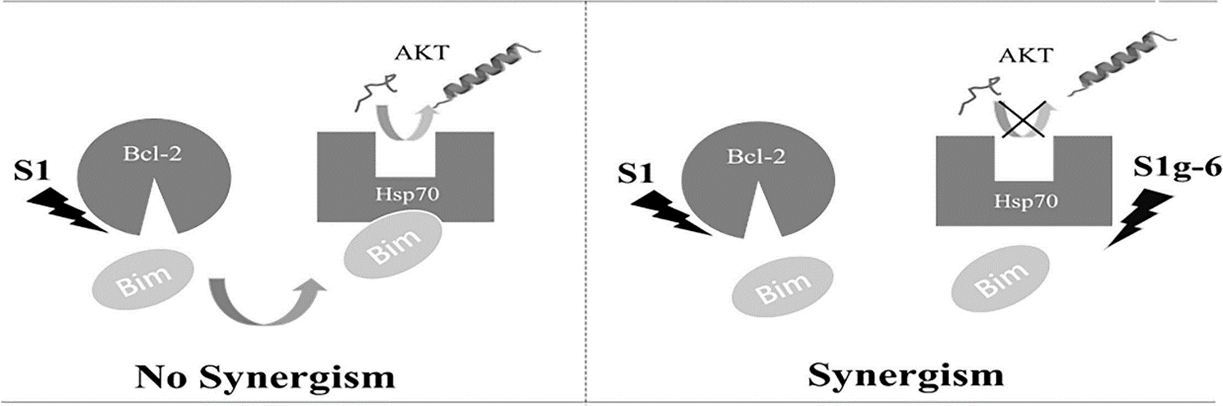
The chaperone heat shock protein 70 (Hsp70) is crucial for avoiding protein misfolding under stress, but it is also upregulated in many kinds of cancers, where its ability to buffer cellular stress prevents apoptosis. Previous research has suggested that Bim, a BH3-only member of the Bcl-2 family proteins, also serves as a cochaperone for Hsp70, which modulates the folding and stabilization of many Hsp70 oncogenic substrates in tumor cells. However, a definitive demonstration of crosstalk between Bcl-2 and Hsp70 family proteins and molecular mechanism remain unclear. Herein, we examined the effects of pan-Bcl-2 inhibitor S1, Hsp70 inhibitor S1g-6 on the K562, U937, H23, HL-60 cell lines and these inhibitors synergistically induce mitochondrial apoptosis in cancer cell lines. Moreover, we identified that Bim transfer between Bcl-2-like protein and Hsp70 underlines Bcl-2/Hsp70 crosstalk in mitochondrial apoptosis pathway. Thus, the synergy of S1 and S1g-6 to induce a panel of cancer cell lines apoptosis by inhibiting free Bim and facilitating oncogenic client AKT folding and activation. Together, our results demonstrated the combination of Bcl-2 inhibitor and Hsp70 inhibitor showed synergistic effect in cancer cells and the potential to decrease tumor regression.
https://www.sciencedirect.com/science/article/pii/S0006295221002732