
Journal of Medicinal Chemistry:
Discovery of Biphenyl Derivatives to Target Hsp70-Bim Protein-Protein Interaction in Chronic Myeloid Leukemia by Scaffold Hopping Strategy
Authors: Maojun Jiang, Hong Zhang, Yang Song, Fangkui Yin, Zhiyuan Hu, Xin Li, Yuying Wang, Zheming Wang, Yitong Li, Zihan Wang, Yanxin Zhang, Siyao Wang, Shaohua Lu, Guanghong Xu, Ting Song, Ziqian Wang*, and Zhichao Zhang*
Abstract
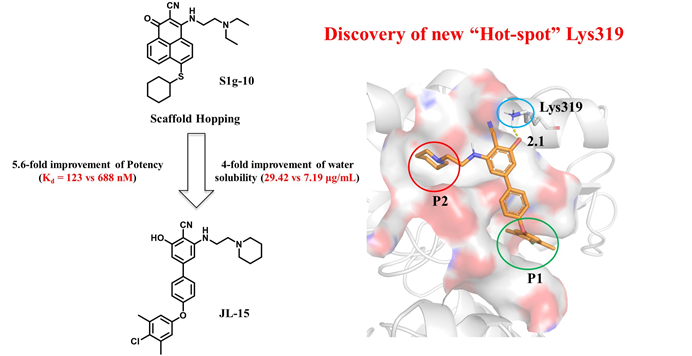
Hsp70-Bim protein–protein interaction (PPI) is the most recently identified specific target in chronic myeloid leukemia (CML) therapy. Herein, we developed a new class of Hsp70-Bim PPI inhibitors via scaffold hopping of S1g-10, the most potent Hsp70-Bim PPI inhibitor thus far. Through structure–activity relationship (SAR) study, we obtained a biphenyl scaffold compound JL-15 with a 5.6-fold improvement in Hsp70-Bim PPI suppression (Kd = 123 vs 688 nM) and a 4-fold improvement in water solubility (29.42 vs 7.19 μg/mL) compared to S1g-10. It maintains comparable apoptosis induction capability with S1g-10 against both TKI-sensitive and TKI-resistant CML cell lines in an Hsp70-Bim-dependent manner. Additionally, through SAR, 1H–15N TRSOY-NMR, and molecular docking, we revealed that Lys319 is a “hot spot” in theHsp70-Bim PPI interface.
Collectively, these results provide a novel chemical scaffold and structural insights for the rational design of Hsp70-Bim PPI inhibitors.
https://pubs.acs.org/doi/10.1021/acs.jmedchem.4c00780