
Journal of Medicinal Chemistry: Bcl-2/MDM2 Dual Inhibitors Based on Universal Pyramid-Like α-Helical Mimetics
Authors: Wang, Ziqian; Song, Ting; Feng, Yingang; Guo, Zongwei; Fan, Yudan; Xu, Wenjie; Liu, Lu; Wang, Anhui; Zhang, Zhichao*
Abstract
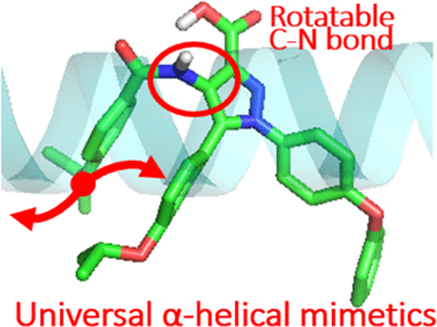
No α-helical mimetic that exhibits Bcl-2/MDM2 dual inhibition has been rationally designed due to the different helicities of the α-helixes at their binding interfaces. Herein, we extracted a one-turn α-helix-mimicking ortho-triarene unit from o-phenylene foldamers. Linking benzamide substrates with a rotatable C–N bond, we constructed a novel semirigid pyramid-like scaffold that could support its two-turn α-helix mimicry without aromatic stacking interactions and could adopt the different dihedral angles of the key residues of p53 and BH3-only peptides. On the basis of this universal scaffold, a series of substituent groups were installed to capture the key residues of both p53TAD and BimBH3 and balance the differences of the bulks between them. Identified by FP, ITC, and NMR spectroscopy, a compound 6e (zq-1) that directly binds to Mcl-1, Bcl-2, and MDM2 with balanced submicromolar affinities was obtained. Cell-based experiments demonstrated its antitumor ability through Bcl-2/MDM2 dual inhibition simultaneously.
https://doi.org/10.1021/acs.jmedchem.5b01913