
Biochemical Pharmacology: Bcr-Abl drives the formation of Hsp70/Bim PPI to stabilize oncogenic clients and prevent cells from undergoing apoptosis
Authors: Hong Zhang , Ting Song , Ziqian Wang , Uwituze Laura Bonnette , Yafei Guo , Hang Wang , Qishuang Gao , Zhichao Zhang
Abstract
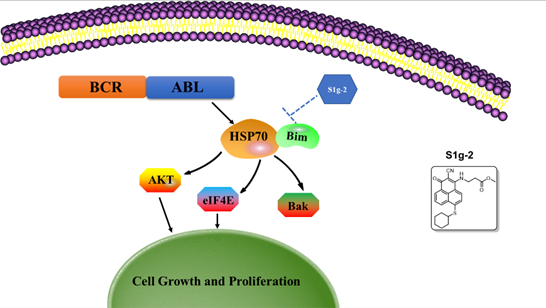
Although tyrosine kinase inhibitors that inhibit Bcr-Abl kinase activity have shown excellent efficacy in the clinical application of CML patients, it still a challenge to discover alternative targets and novel therapies because of the emergence of Bcr-Abl-independent resistance. Most recently, Hsp70/Bim complex was revealed that driven by Bcr-Abl and testified as a specific target for CML because it folds and stabilizes many Hsp70 oncogenic substrates that mediate CML specific signaling pathways. However, the relationship between Bcr-Abl and Hsp70/Bim complex and how the chaperone complex contributes to Bcr-Abl-driven leukemogenic cells remain unclear. Herein, with the help of S1g-2, a specific small-molecule inhibitor of Hsp70/Bim complex, and Bcr-Abl knockdown to induce a panel of cancer cell lines apoptosis, we illustrated that Bcr-Abl could prevent cells from undergoing apoptosis mainly by driving the formation of Hsp70/Bim complex both in Bcr-Abl positive CML cells and ALL cells. Through cell-based Co-immunoprecipitation experiments, we identified that Bcr-Abl could stabilize oncogenic clients including AKT and eIF4E mainly by driving the formation of Hsp70/Bim complex in Bcr-Abl positive cells. Moreover, we identified that Bim mediates interactions of Hsp70 and Bak in Bcr-Abl positive cells. Together, the target identification of Hsp70/Bim complex could make it as a promising anticancer target for Bcr-Abl positive leukemia treatment.
https://doi.org/10.1016/j.bcp.2022.114964